Lessons in Gait from Autistic Kids
/“Additionally, there is the potential for the cerebellum, which receives sensory information and regulates movements, to have a level of dysfunction as well. Viewed collectively, the potential key contributors for gait asymmetry originate in the brain and specifically, the motor-controlling functions of the brain.”
“While there is still little known regarding gait impairments in children with ASD, our findings illustrate that gait descriptors may provide insight into furthering working knowledge of ASD and may even enable gait-related symptoms to be treatable through therapies and interventions”
“Alternative hypotheses suggest that children with ASD exhibit dysfunctional segregation of the motor cortex, which may be the key to uncoordinated movements”
We often say that "gait is a fingerprint". Gait symmetry is often considered a window to neurologic function. We like to think "normal" gait has minimal asymmetries, while pathological gait does not.
These are two landmark studies of gait in children with autism spectrum disorder. There were significant kinetic and kinematic differences in gait patterns in the 3 cardinal planes (saggital, coronal and transverse) in ankle, knee and hip mechanics: The "pattern" is that there is no pattern, only changes. If you have a little time, check out this free, full text article here.
What this article says to us is that
- We should be looking more carefully at gait asymmetries realizing that
- These asymmetries are most likely cortical/cerebellar phenomena implying
- Gait dysfunction equals cortical/cerebellar dysfunction
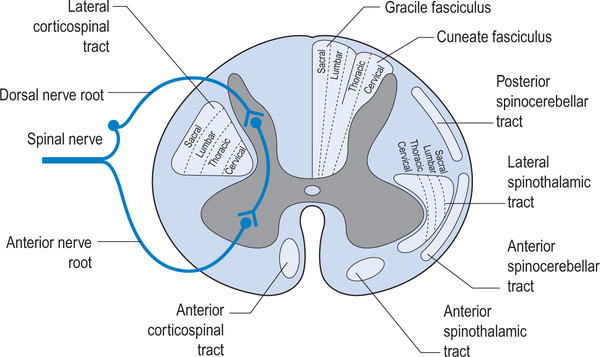
As clinician's, we should be thinking of altered gait as a window to what is going on north of the feet, knees, hip and pelvis. We remember that the joint and muscle mechanoreceptors feedback to the cerebellum and cortex via the spinocerebellar and dorsal column pathways which feed forward to the lower extremities via the anterior spinous cerebellar pathway as well as cortical spinal, rubrospinal had vestibula spinal pathways. The cortex, particularly the motor portion, has the capacity to alter gait just as abnormal mechanoreception has the capacity to alter cortical and cerebellar function. The two are interrelated and inseparable. Changes over time will altered pathways due to neural plasticity and adaptations will occur.
We need to be prudent and examined people fully and be very careful as to the modalities and exercises that we utilize and prescribed as ultimately they will shape that patients neural architecture.
Eggleston JD, Harry JR, Hickman R, Dufek JS. Analysis of gait symmetry during overground walking in children with autism spectrum disorder. Gait Posture 2017;55:162-166.
Dufek JS, Eggleston JD, Harry JR, Hickman R. A comparative evaluation of gait between children with autism and typically developing matched controls. Med Sci 2017;5:1. link to free full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5635776/