Better gait AFTER rhizotomies?
/Nothing surprised me more than reading this paper and finding out that folks that have had rhizotomies, which removes the afferent input from the dorsal horn and sensory information from the reflex loops in the cord, actually had better gait. Of course these children had severe spastic diplegia, which means they have lost descending inhibition from higher center's and most likely had increased flexor tone in the lower extremities.
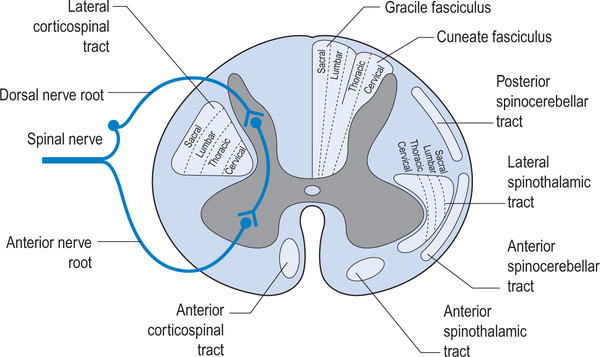
image credit: http://realtyconnect.me/spinal-cord-cross-section-tracts/background-information-musculoskeletal-key-within-spinal-cord-cross-section-tracts/
Remember that the fibers entering the dorsal horn not only go to the dorsal columns but also to the spinocerebellar pathways. When someone has spasticity, the feedback loops are skewed and flexor drive coming from the rostral reticular formation generally is increased are often kept in check by the cerebellar and vestibular feedback loops. Perhaps the interruption of this feedback loop and lack of information from type IA and II afferents of the muscle spindles as well as Ib afferents from the globe tendon organs modulated the tone sufficiently to improve gait. This study did a selective dorsal rhizotomy which means only a portion of it was ablated.
The somatotopic organization of the dorsal horn of the spinal cord (i.e.: certain areas of the dorsal horn correspond to certain body parts) is well documented in humans; It would make sense that the dorsal root itself (i.e.: the afferent fibers in the nerve going into the dorsal horn) would be as well, as they are that way in murines (2) and felines (3).
So, how does this apply to gait? People with strokes, cortical lesions, diseases like cerebral palsy and even possibly increased flexor tone, may benefit from altered input into the dorsal horn. It would have been really cool to see if they increased extensor activity in this individuals, if they would be benefited further.
Abstract
OBJECTIVE: To identify factors associated with long-term improvement in gait in children after selective dorsal rhizotomy (SDR).
DESIGN: Retrospective cohort study SETTING: University medical center PARTICIPANTS: 36 children (age 4-13y) with spastic diplegia (gross motor classification system level I (n=14), II (n=15) and III (n=7) were included retrospectively from the database of our hospital. Children underwent selective dorsal rhizotomy (SDR) between January 1999 and May 2011. Patients were included if they received clinical gait analysis before and five years post-SDR, age >4 years at time of SDR and if brain MRI-scan was available.
INTERVENTION: Selective dorsal rhizotomy MAIN OUTCOME MEASURES: Overall gait quality was assessed with Edinburgh visual gait score (EVGS), before and five years after SDR. In addition, knee and ankle angles at initial contact and midstance were evaluated. To identify predictors for gait improvement, several factors were evaluated including: functional mobility level (GMFCS), presence of white matter abnormalities on brain-MRI, and selective motor control during gait (synergy analysis).
RESULTS: Overall gait quality improved after SDR, with a large variation between patients. Multiple linear regression analysis revealed that worse score on EVGS and better GMFCS were independently related to gait improvement. Gait improved more in children with GMFCS I & II compared to III. No differences were observed between children with or without white matter abnormalities on brain MRI. Selective motor control during gait was predictive for improvement of knee angle at initial contact and midstance, but not for EVGS.
CONCLUSION: Functional mobility level and baseline gait quality are both important factors to predict gait outcomes after SDR. If candidates are well selected, SDR can be a successful intervention to improve gait both in children with brain MRI abnormalities as well as other causes of spastic diplegia.
1. Oudenhoven LM, van der Krogt MM, Romei M, van Schie PEM, van de Pol LA, van Ouwerkerk WJR, Harlaar Prof J, Buizer AI. Factors associated with long-term improvement of gait after selective dorsal rhizotomy. Arch Phys Med Rehabil. 2018 Jul 4. pii: S0003-9993(18)30442-8. doi: 10.1016/j.apmr.2018.06.016. [Epub ahead of print]
2. Wessels WJ1, Marani E. A rostrocaudal somatotopic organization in the brachial dorsal root ganglia of neonatal rats. Clin Neurol Neurosurg. 1993;95 Suppl:S3-11.
3. Koerber HR, Brown PB. Somatotopic organization of hindlimb cutaneous nerve projections to cat dorsal horn. J Neurophysiol. 1982 Aug;48(2):481-9.